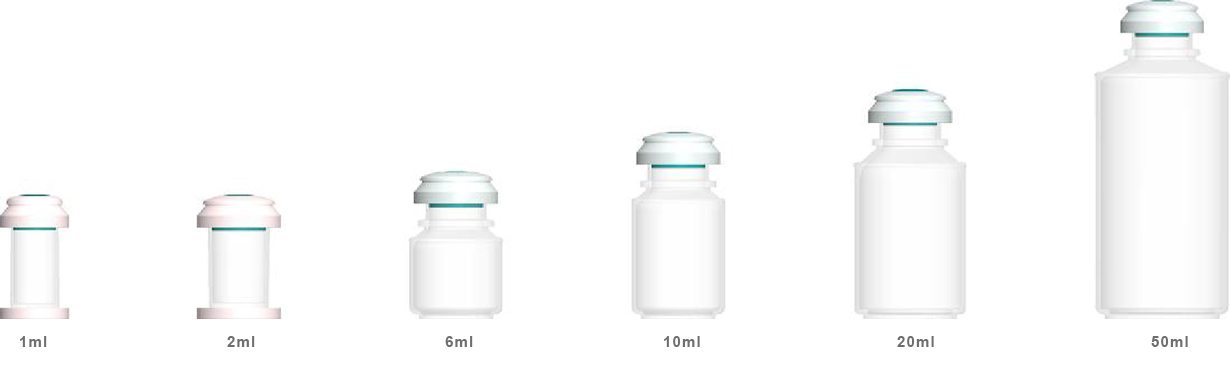
技術概念 / Concept
The AT-Closed Vial® Technology is based on the concept of the ready-to-fill Closed Vial, whereby polymer vials are provided:
-clean (molded in ISO 5 clean room);
-closed (stopper in place and secured);
-sterilized (gamma-irradiated).
應用領域 / Fields of use
 Biological
Biological
-Vaccines
-mAbs
-RNA/DNA-based products
Low leachable profile, twice less particles then in glass vial. Potent
Potent
-Radiopharmaceuticals
-mAbs
-Nanomedicines
Unbreakable container, always closed to avoid contamination. Cryostored
Cryostored
-Cell therapy
-Gene therapy
-Other low T° storage products
Container Closure Integrity even in liquid nitrogen.
步驟說明 / Process
The overall filling process of the ready-to-fill AT-Closed Vials® is made of very few steps:
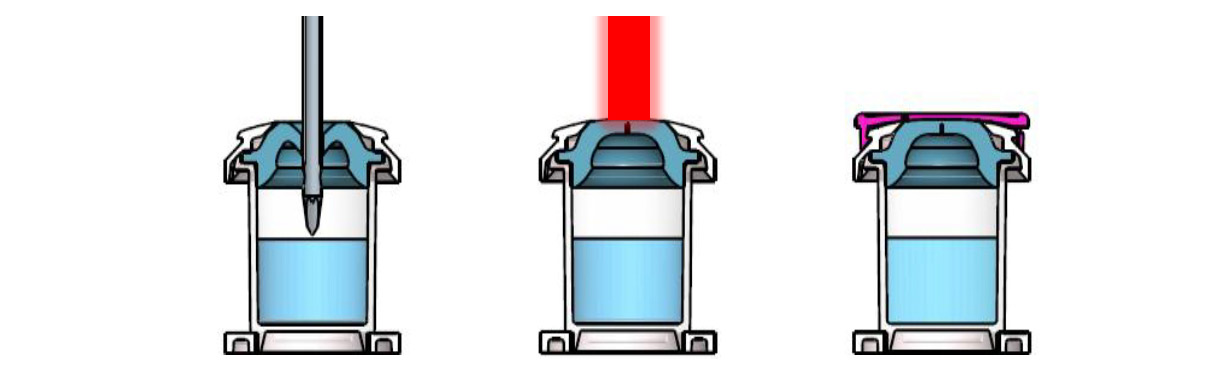
1.Filling of the vial with a needle puncturing the elastomeric stopper.
2.Immediate laser re-sealing to restore the closure integrity of the filled vial.
3.Capping with a tight snap-fit cap protecting the piercing area.
材質 / Materials
Materials selected for the product contact parts meet USP and EP requirements for pharmaceutical primary container:
-COC (Cyclo-Olefin Co-polymer) for vial body, known for shock-resistance, barrier properties and transparency;
-TPE (Thermo Plastic Elastomer) for stopper, a proprietary material allowing specific processing;
both having excellent leachable profile.
The fully automated vial manufacturing process ensures clean conditions: vial and stopper are molded in Grade A/ISO 5 and immediately assembled by robots, minimizing particle content compared to other types of primary containers.
AT-Closed Vials® are then packed and sterilized by gamma irradiation, being supplied as Ready-to-Fill containers.
.jpg)
.jpg)